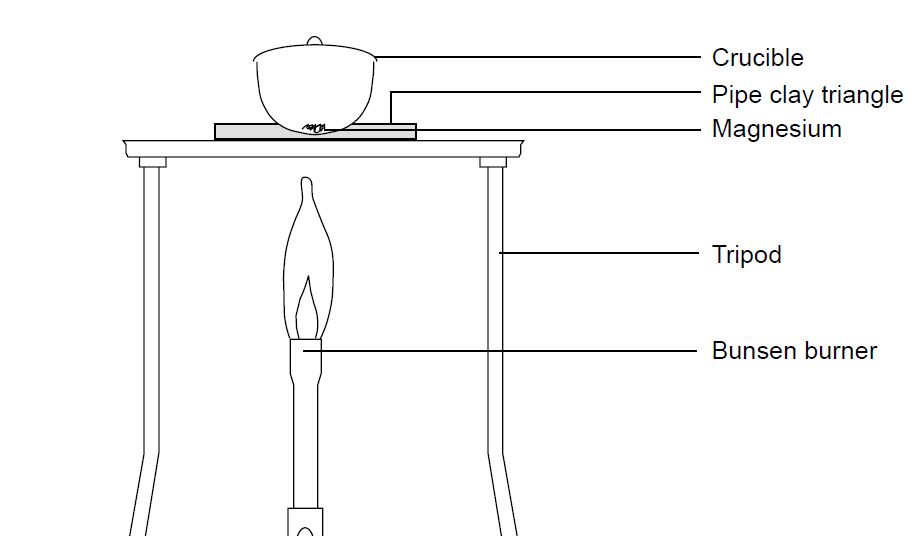
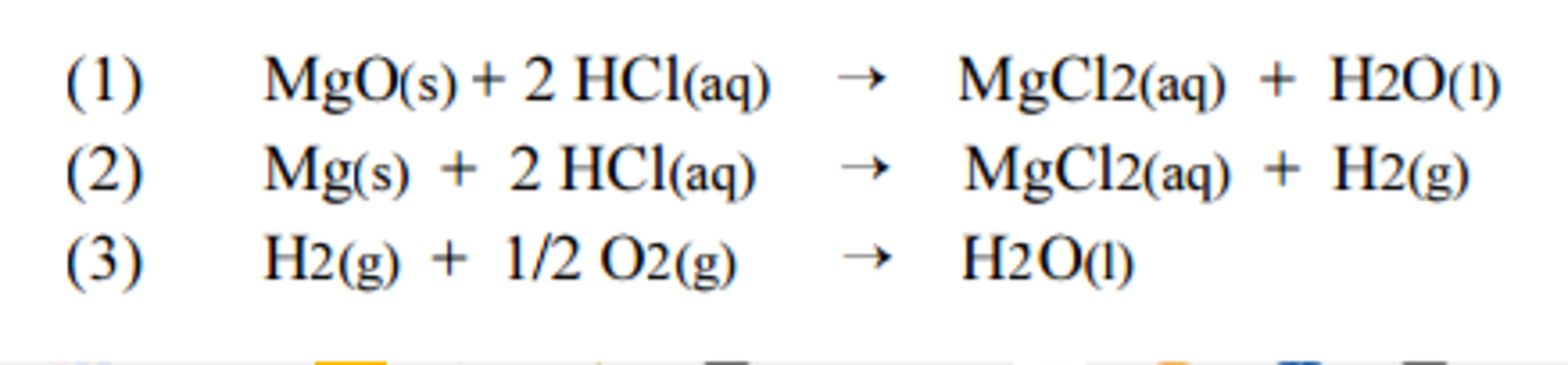Skills Lab: Combination of Matter Essential Question: When I burn something, does it get heavier or lighter? Mg + O ppt download
Burning magnesium in a Bunsen flame and other flame experiments | Chem 13 News Magazine | University of Waterloo
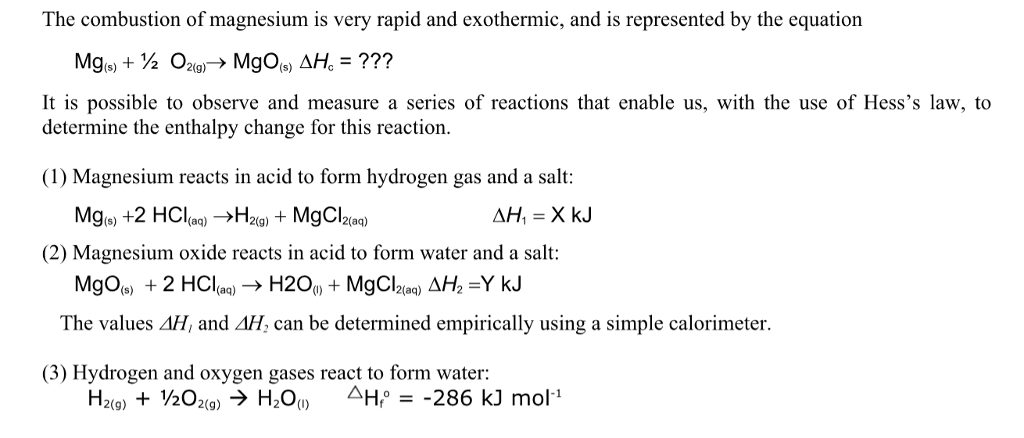
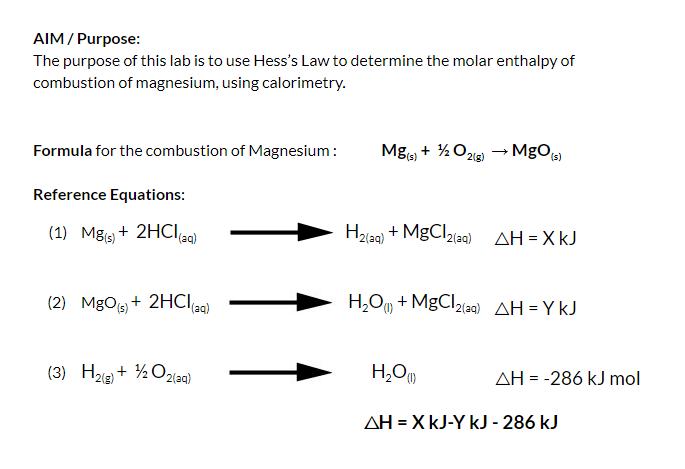
![SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)- SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)-](https://cdn.numerade.com/ask_previews/1bda9caf-ebe2-4ef5-bcb8-b82a12a7fae5_large.jpg)
SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)-
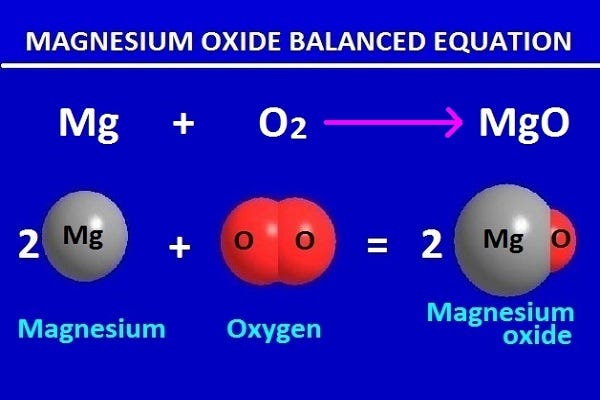
Magnesium oxide balanced equation in chemistry for class 9 | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

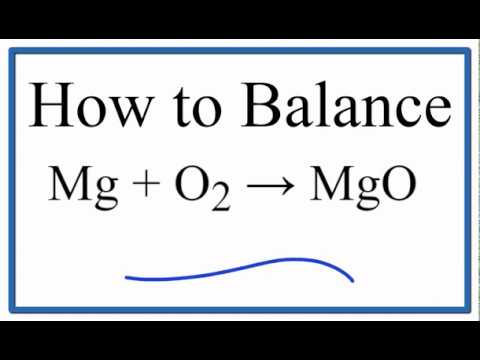
Write a balanced chemical equation for the following chemical reaction : Magnesium burns in oxygen - YouTube
Magnesium and carbon dioxide – Student sheet Burning magnesium in carbon dioxide – what will happen? To do
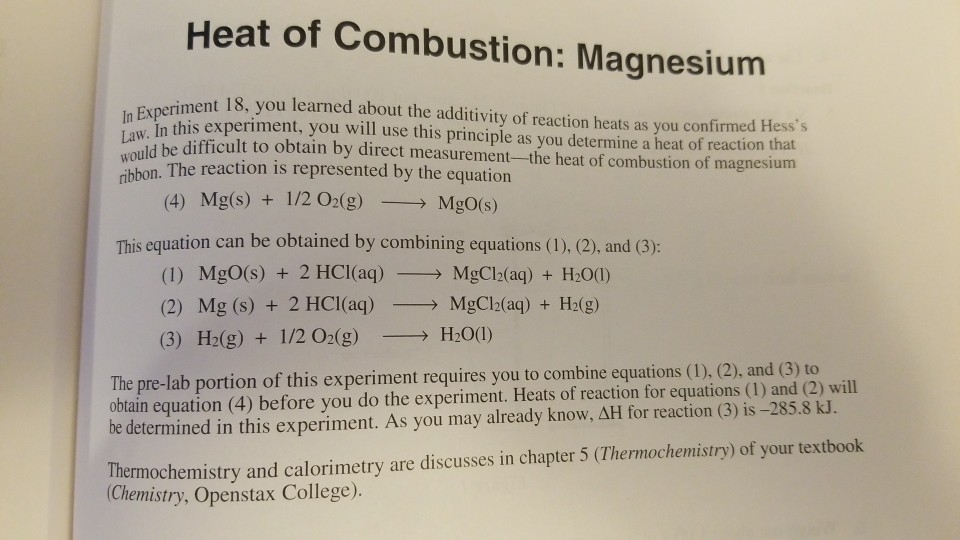
![SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)- SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)-](https://cdn.numerade.com/ask_images/f4ecd30e9ac046f595968ff9727743c4.jpg)
SOLVED: 1) The combustion of magnesium metal is represented by the equation below: Mg(s)+%z 02(g) MgO(s) Determine the molar enthalpy of combustion of magnesium, using your data and Hess's Law[12] Mg(s)+ 2HClaq)-