Takeda's tetravalent dengue vaccine (TAK-003) receives first global approval for use in Indonesia without need for pre-vaccination testing | Asia Research News

Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination. - Abstract - Europe PMC
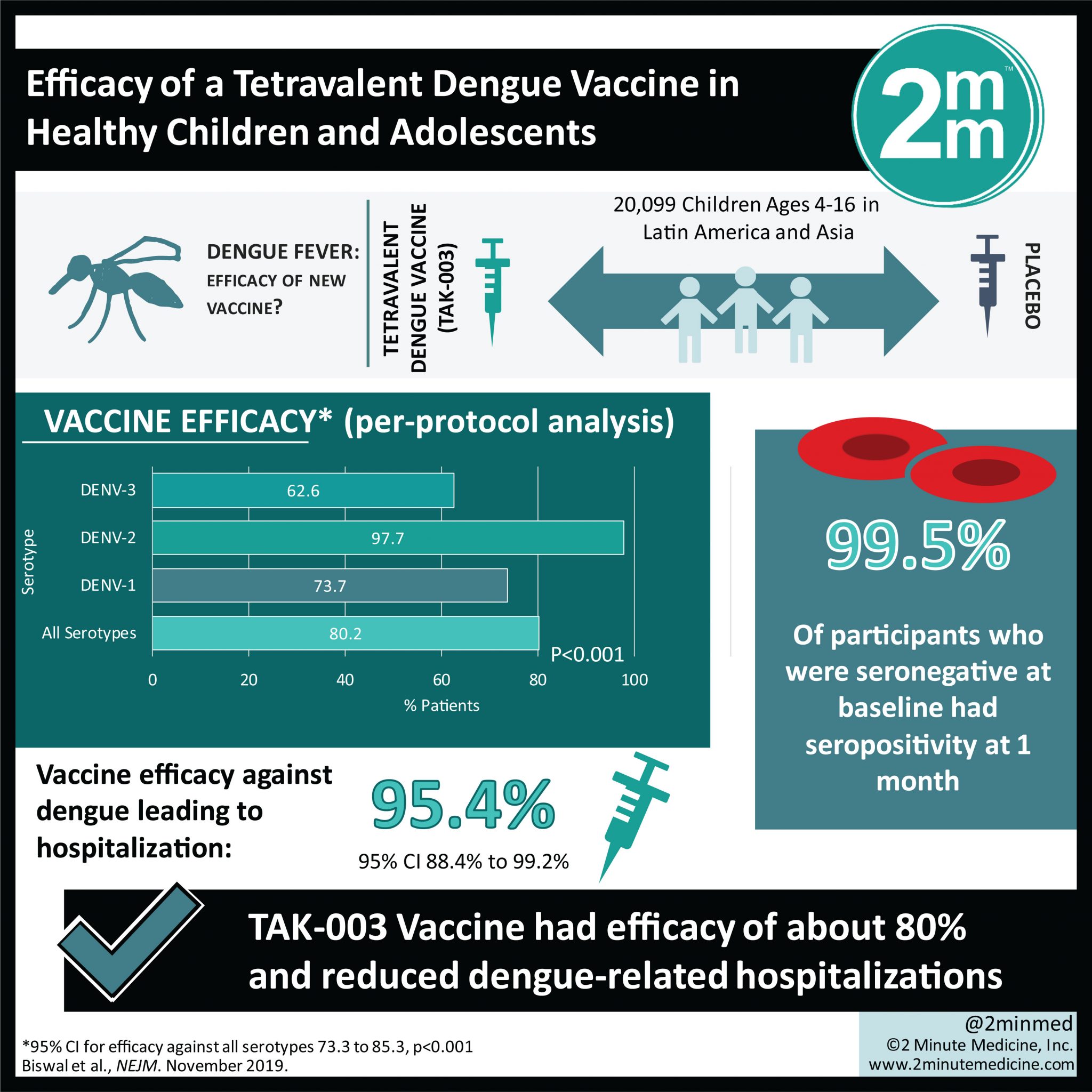
VisualAbstract: Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents | 2 Minute Medicine

Takeda dengue vaccine TAK-003 provides continued protection against dengue fever through 4.5 years in trial
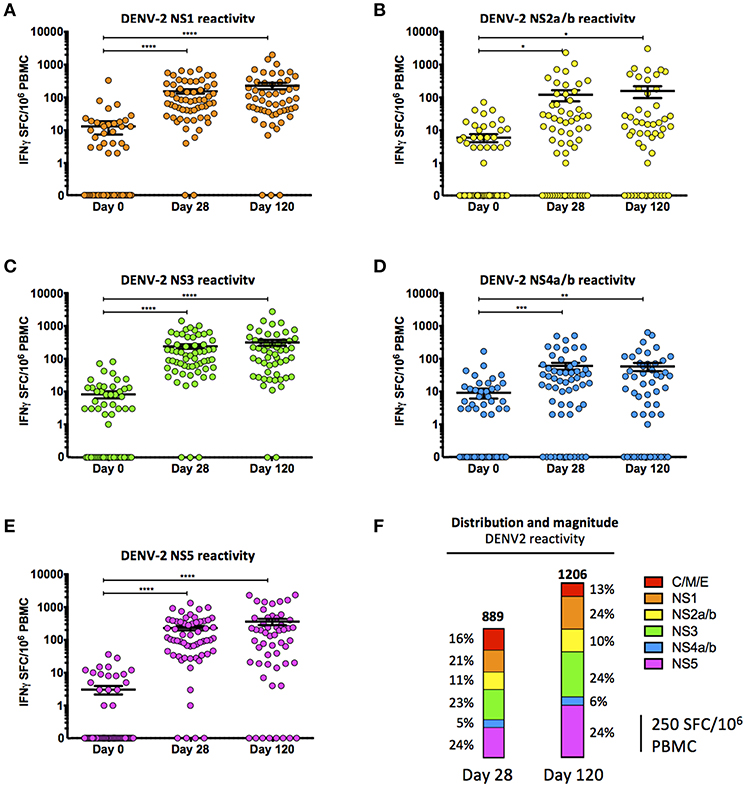
Frontiers | Assessing the Diversity and Stability of Cellular Immunity Generated in Response to the Candidate Live-Attenuated Dengue Virus Vaccine TAK-003
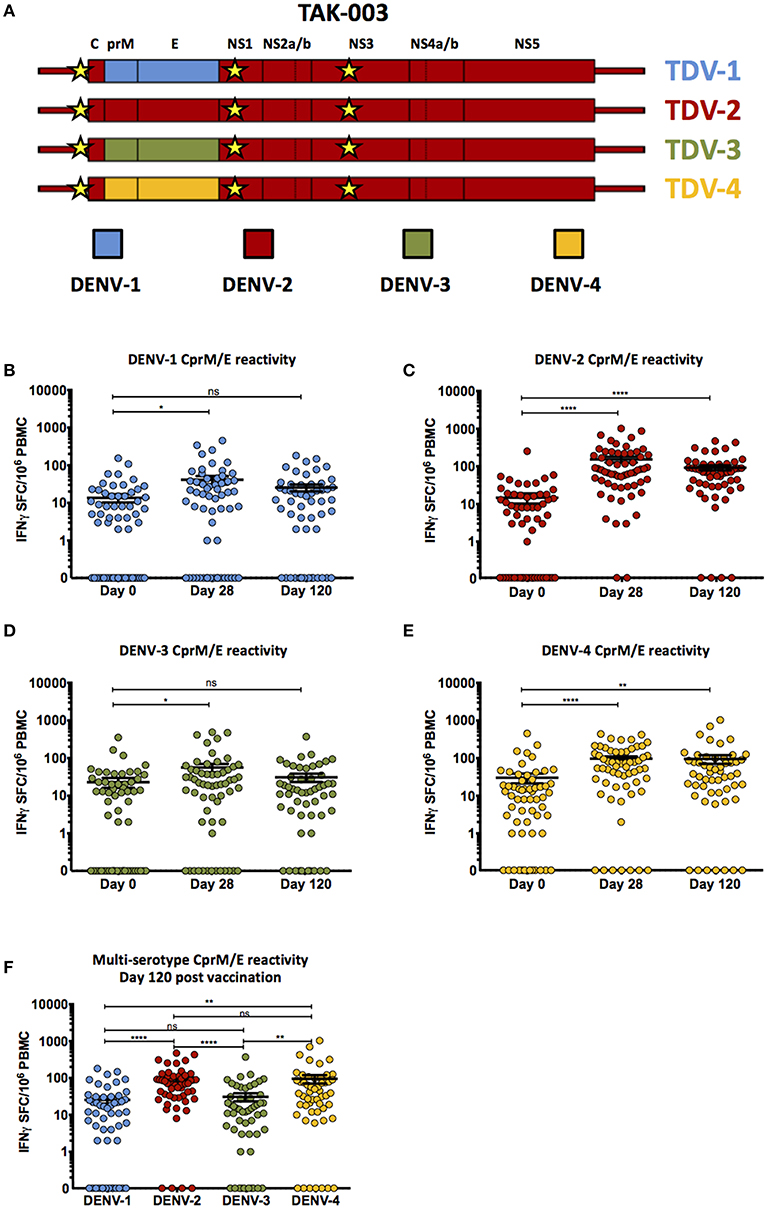
Frontiers | Assessing the Diversity and Stability of Cellular Immunity Generated in Response to the Candidate Live-Attenuated Dengue Virus Vaccine TAK-003

Takeda's Biologics License Application (BLA) for Dengue Vaccine Candidate ( TAK-003) Granted Priority Review by U.S. Food and Drug Administration - Headlines of Today

Takeda's dengue shot keeps 84% of kids out of hospital regardless of prior infection, giving it a leg up over Sanofi's Dengvaxia | Fierce Biotech
Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (TAK-003) | PLOS Neglected Tropical Diseases

Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination. - Abstract - Europe PMC
Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (TAK-003) | PLOS Neglected Tropical Diseases

Takeda Dengue vaccine TAK-003 provides continued protection against dengue fever through 4.5 years in trial

PharmaShots. - • The submission is based on a P-III TIDES trial assessing TAK-003 (0.5ml, SC) vs PBO in 20,000+ healthy children & adolescents aged 4-16yrs. to prevent dengue fever of any

Potential Impact of Takeda's Dengue Vaccine Candidate Reinforced by Long-Term Safety and Efficacy Results