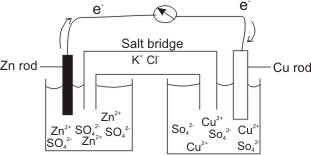
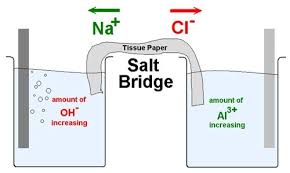
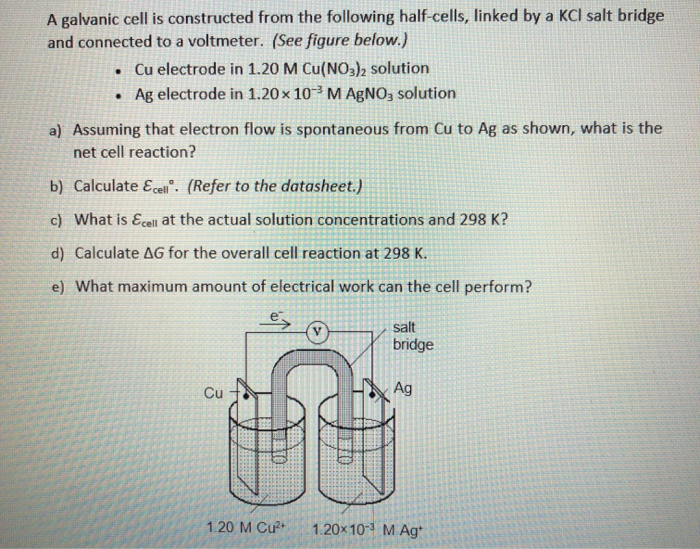
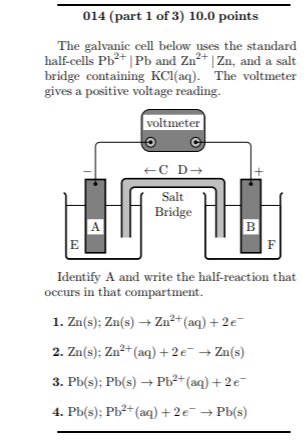
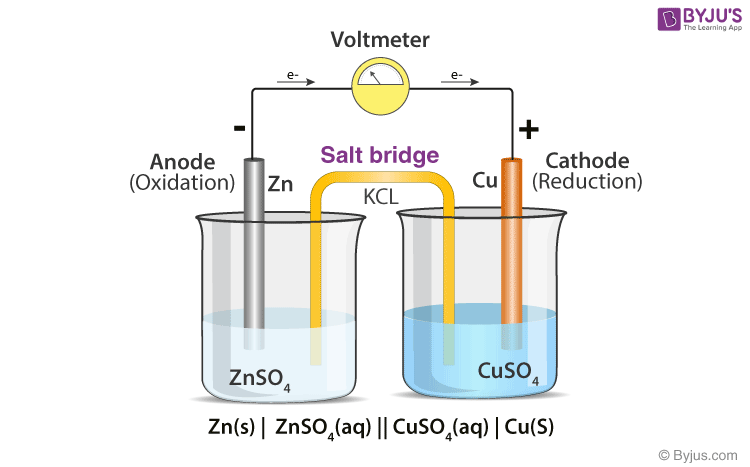
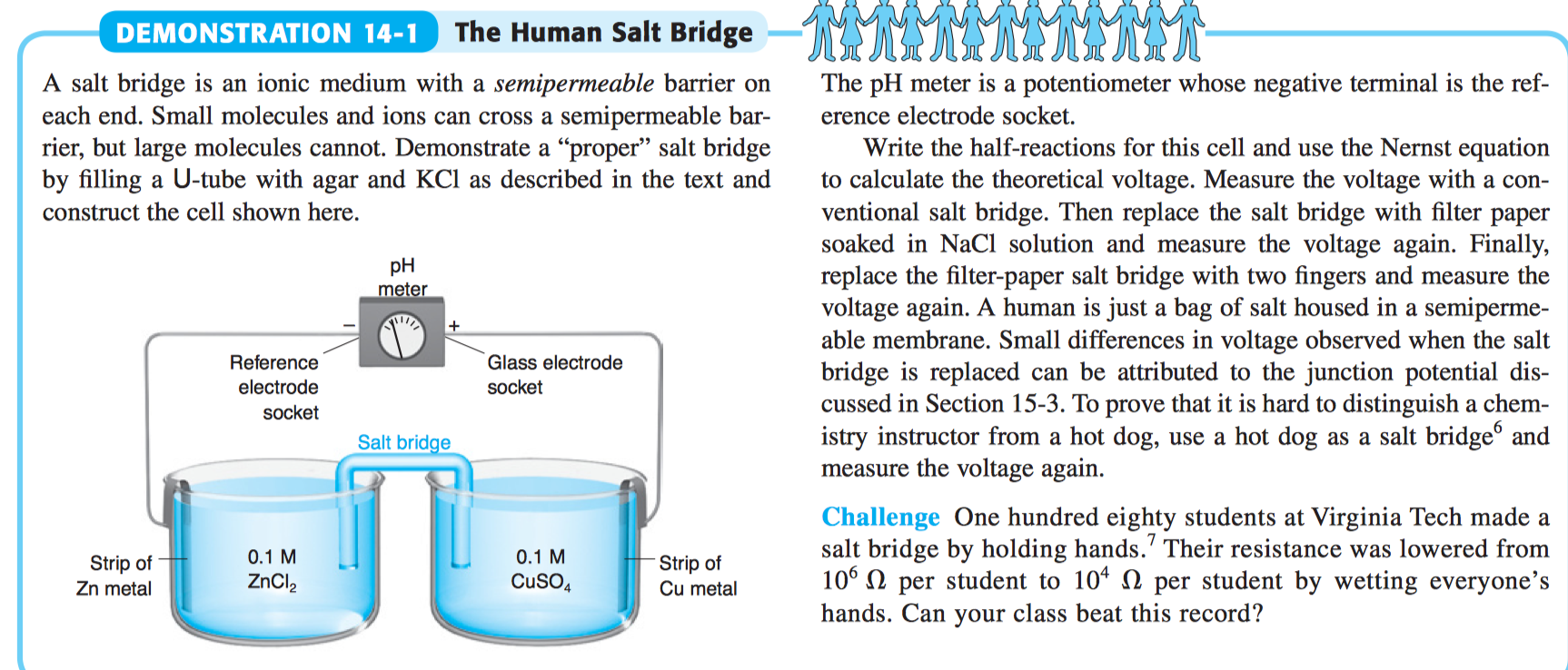
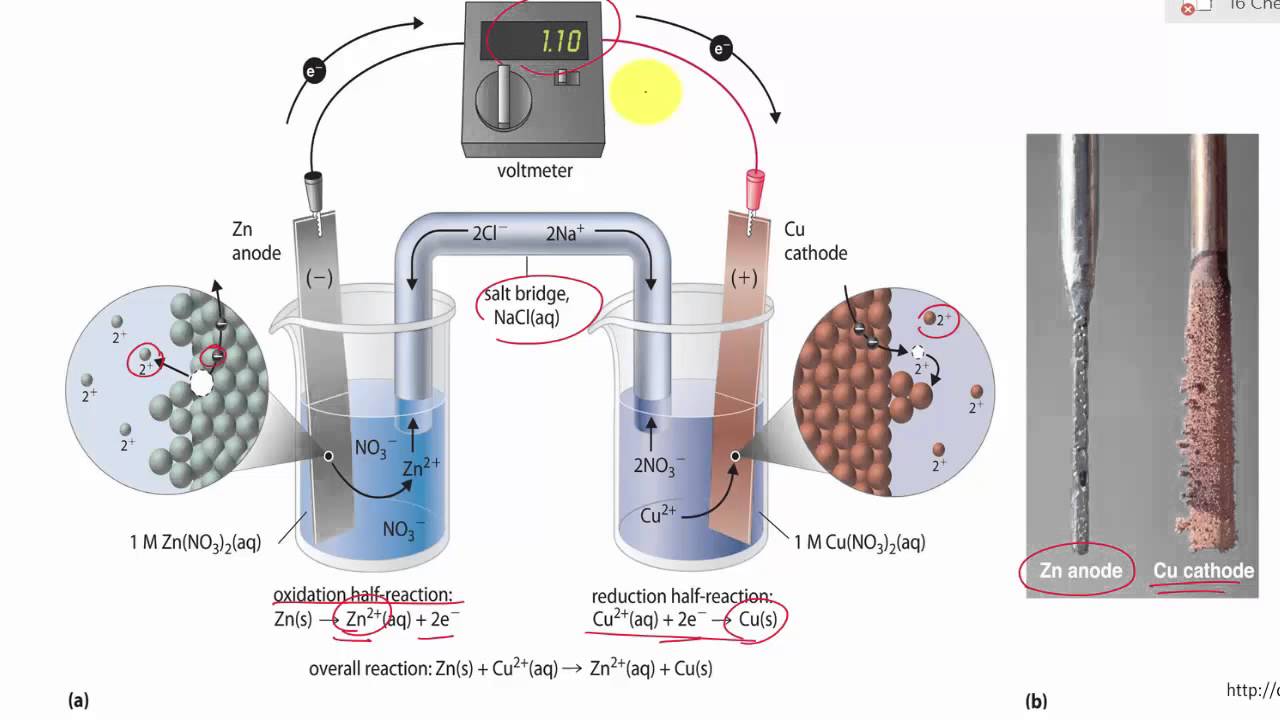
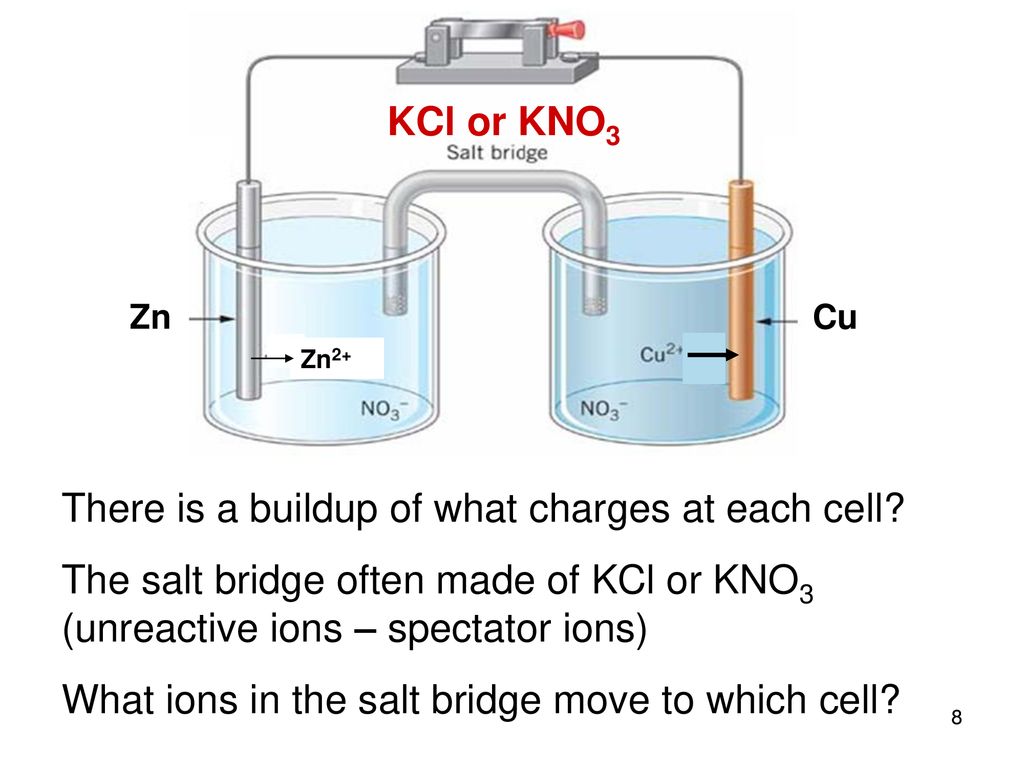
A salt bridge is used in voltaic cells to balance the ions and complete the circuit. Describe this scenario. (hint;use a diagram) | Homework.Study.com
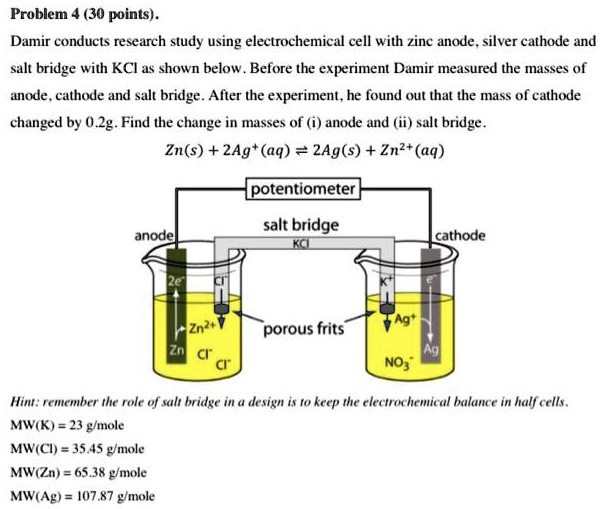
SOLVED: Problem 4 (30 points) . Damir conducts research study using electrochemical cell with zine anode silver cathode und salt bridge with KCl as shown below. Before the experiment Damir measured the

A Micro-agar Salt Bridge Electrode for Analyzing the Proton Turnover Rate of Recombinant Membrane Proteins. | Semantic Scholar

KCl cannot be used as a salt bridge for the cell Cu(s) abs(CuSO(4)(aq))abs(AgNO(3)(aq)) Ag(s) because:

Schematic Diagram of a Potentiometric Electrochemical Cell | Image and Video Exchange ForumImage and Video Exchange Forum

A) Schematic of the micro-agar salt bridge. Three percent agarose in 3... | Download Scientific Diagram
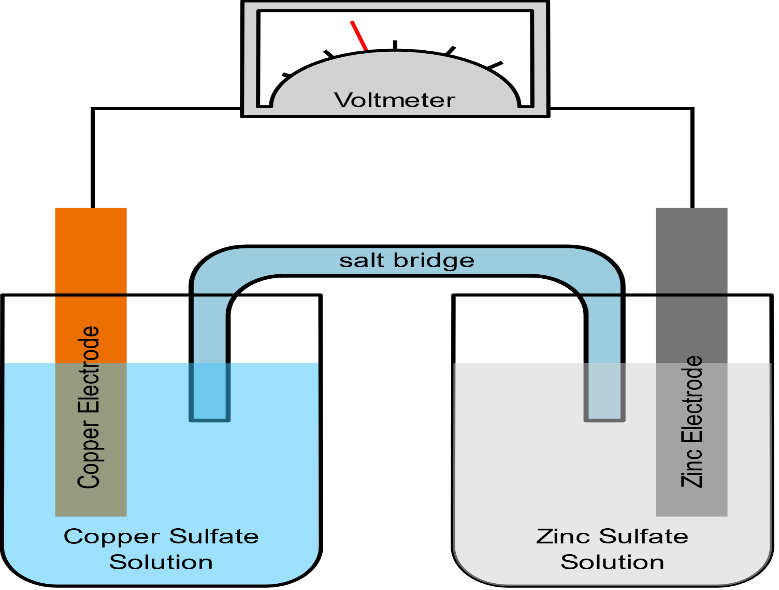
The function(s) of salt bridge in a cell is\/areA. It maintains standard electrode potential of cell constant which depends on several factors.B. It completes the electrical circuit.C. It departs both the solutions