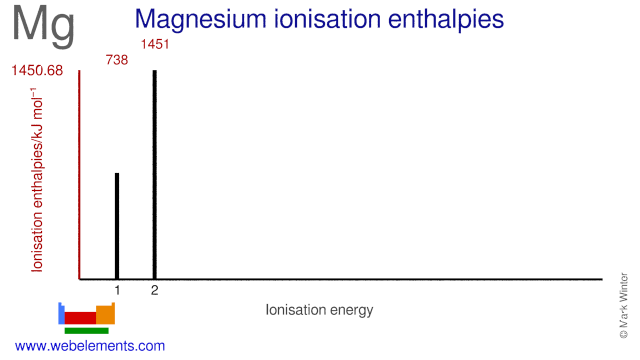
inorganic chemistry - Why does the ionization energy decrease anytime the atom size increases? - Chemistry Stack Exchange
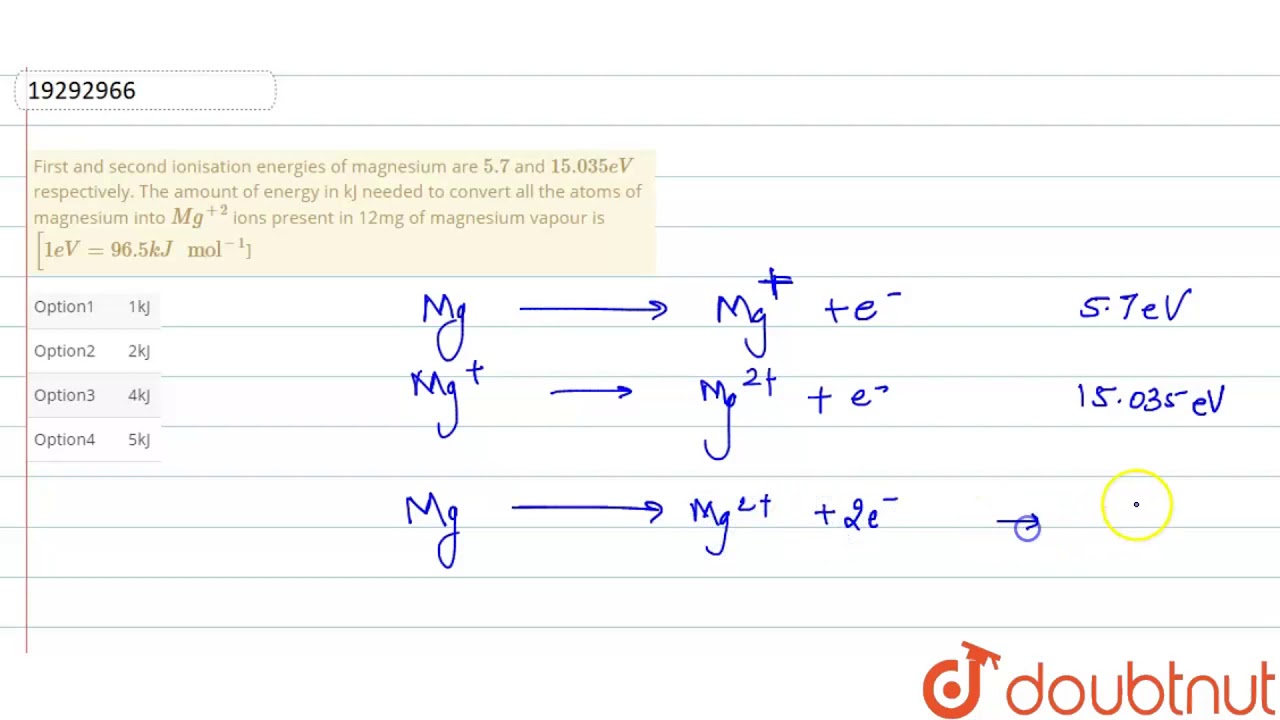
First and second ionisation energies of magnesium are `5.7` and `15.035eV` respectively. The amount - YouTube

Ionisation Energies: Electronic Configuration (1.2.10) | CIE AS Chemistry Revision Notes 2019 | Save My Exams
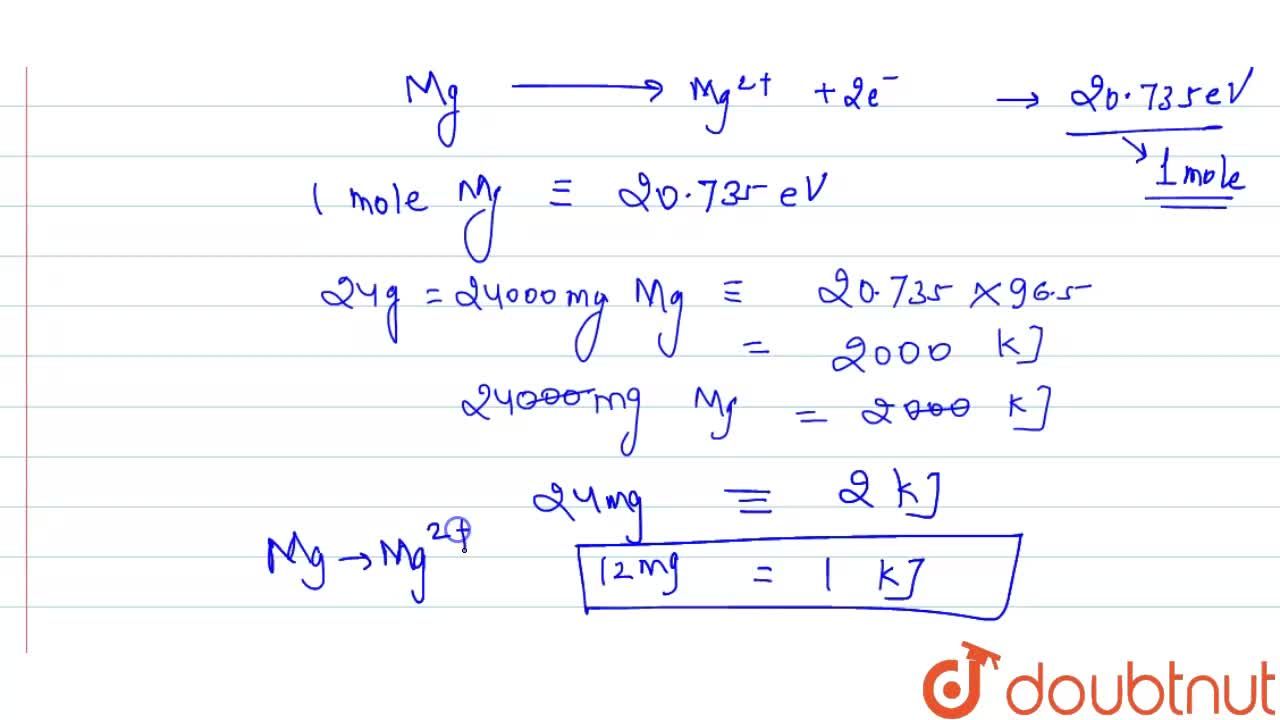
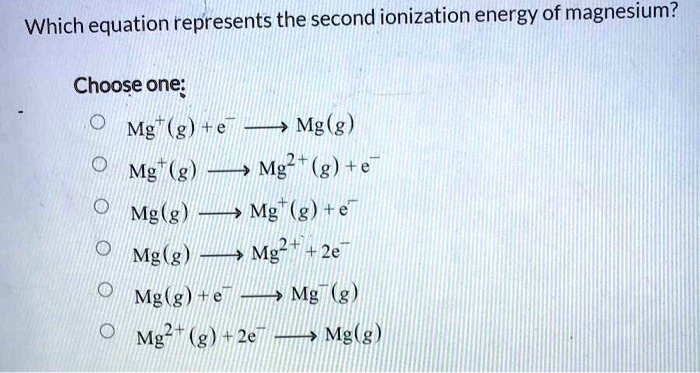
First and second ionisation energies of magnesium are 5.7 and 15.035eV respectively. The amount of energy in kJ needed to convert all the atoms of magnesium into Mg^(+2) ions present in 12mg

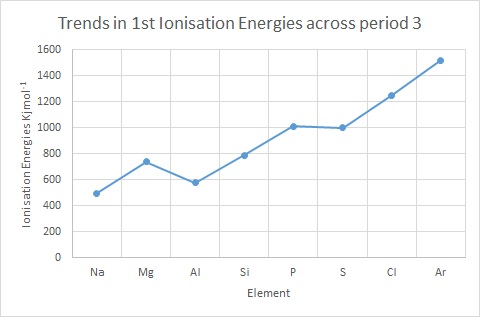
Ionisation Energy: Trends & Evidence (1.1.7) | AQA A Level Chemistry Revision Notes 2017 | Save My Exams

First and second ionisation energies of magnesium are `7.646 eV` and `15.035ev` respectively. - YouTube

First and second ionisation energies of magnesium are 7.646 and 15.035eV respectively. The amount of energy in kJ needed to convert all the atoms of magnesium into Mg ^2 + ions present

Magnesium Chemical Element First Ionization Energy Stock Vector (Royalty Free) 1230396949 | Shutterstock
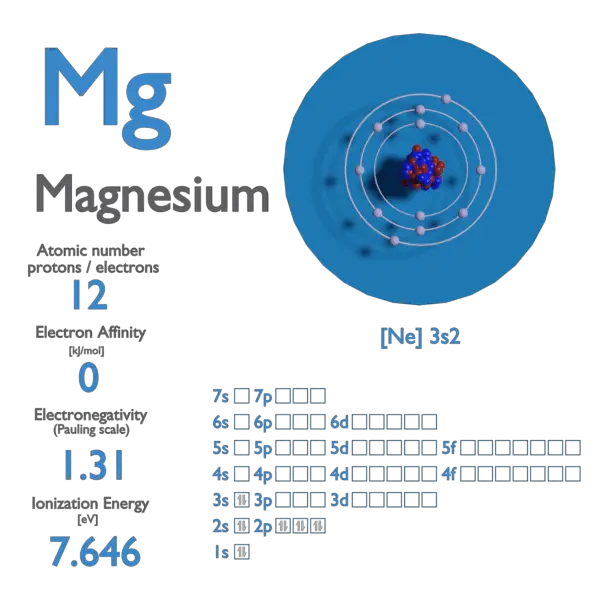
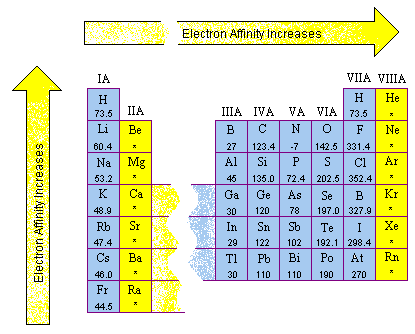
Magnesium - Electron Affinity - Electronegativity - Ionization Energy of Magnesium | nuclear-power.com
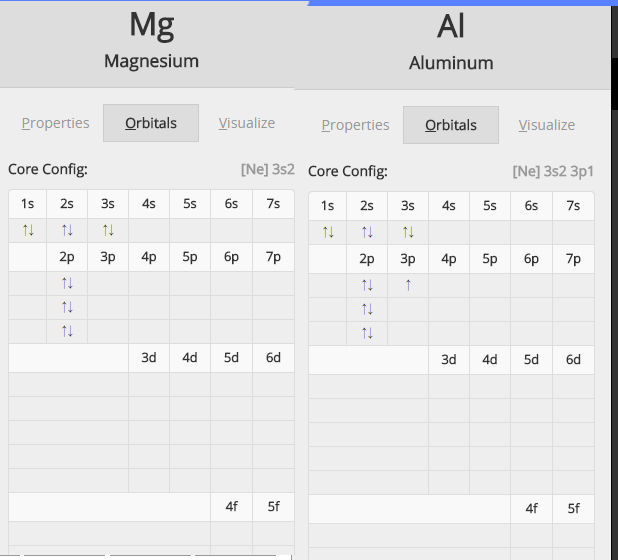
✓ Solved: Writing Exercises Explain why the first ionization energy of magnesium is greater than the...